Developing Covid Vaccine – A Race Against Time7 min read
In India, companies have joined hands to develop a vaccine to contain the deadly Covid virus that has already infected over 86 lakh people in the country. Generally, it takes years of research and testing to get a vaccine ready and produce it on a large scale, but owing to the pandemic, scientists are working tirelessly to launch a vaccine as soon as possible.
Right now, eight pharma majors i.e. Bharat Biotech, Serum Institute of India, Panacea Biotech, Dr. Reddy’s, Indian Immunologicals, Zydus Cadila, Mynvax, and Biological E are working towards Covid-19 vaccine in India.
Some of the indigenous players namely Hester Biosciences, Premas Biotech, Gujarat Biotechnology Research Centre, Auro Vaccines, Gennova Biopharma, and Christian Medical College, Vellore have recently become part of the league. The newly joined players would perform a crucial role in scaling-up the manufacturing of the vaccine.
If an Indian firm becomes a frontrunner in coming up with a vaccine, it would not only aid in speeding up the healthcare process but would also transform the Indian pharma industry’s reputation from being a mass producer of generics to a drug innovator.
Sujay Shetty, Chairman- Partner Oversight Committee- PwC India, reportedly said “India’s very good at the D side, the development side. It’s more like incremental innovation, so in terms of broad innovation or [research], it’s relatively small.”
Before getting into the finer details of the various stages at which each of these firms is, let’s get an understanding of what function a vaccine performs and how it comes into existence.
How does a vaccine work?
Coronavirus has spikes surrounding it that attach to human cells like Velcro and form strong bonds, which causes infections. With the help of a vaccine, a kind of medicine, harmless copies of the virus is introduced into the body in a safe way.
When the body’s immune system comes into contact with the virus and recognizes it as being foreign, it generates antibodies (protective proteins) specific to the disease-causing virus and destroys the virus. This way the body’s immune system remembers the virus and how to kill it.
The next time you are exposed to the same virus, your immune system is able to quickly kill it before it gets a chance to make you ill. In this manner, your body develops resistance power or immunity to the particular virus.
So what does it take to produce a vaccine?
It can take up to a decade to move from the discovery of a vaccine to its development and approval. Here are the five broad stages to produce life-saving vaccines:
Preclinical Stage:
This is a research-intensive stage that aims to find out natural or synthetic antigens that would trigger a similar reaction as the real virus would. Antigens are foreign substances that elicit an immune response in your body.
Phase 1/2a and 2b:
In Phase 1, the vaccine is tested for the first time on a small group of 20-80 adults to assess its safety and gauge the immune system’s response to it. Then, Phase 2a determines the dosage that will be most effective and also aims to enhance the safety experience with the vaccine. Researchers may look for adverse reactions like headache, fever, muscle aches, among others. Phase 2b trial involves administering the vaccine to a larger number of people.
Phase 3:
In this stage, several other volunteers join the clinical trial and receive the vaccine in order to examine whether it is effective or not.
Regulatory approval:
Once the Phase 3 trial becomes successful, the vaccine manufacturing company applies to the regulatory bodies for approval. The regulator reviews the clinical trial data to ensure that the vaccine is safe and effective. After the vaccine gets approved, the pharma company proceeds with its mass production.
Phase 4:
Even post the vaccine approval and licensure stage, the regulator continues to stay involved, monitors its production, inspects manufacturing facilities; and conducts vaccine tests to ensure its potency, safety, and purity.
At which stage are our pharma companies?
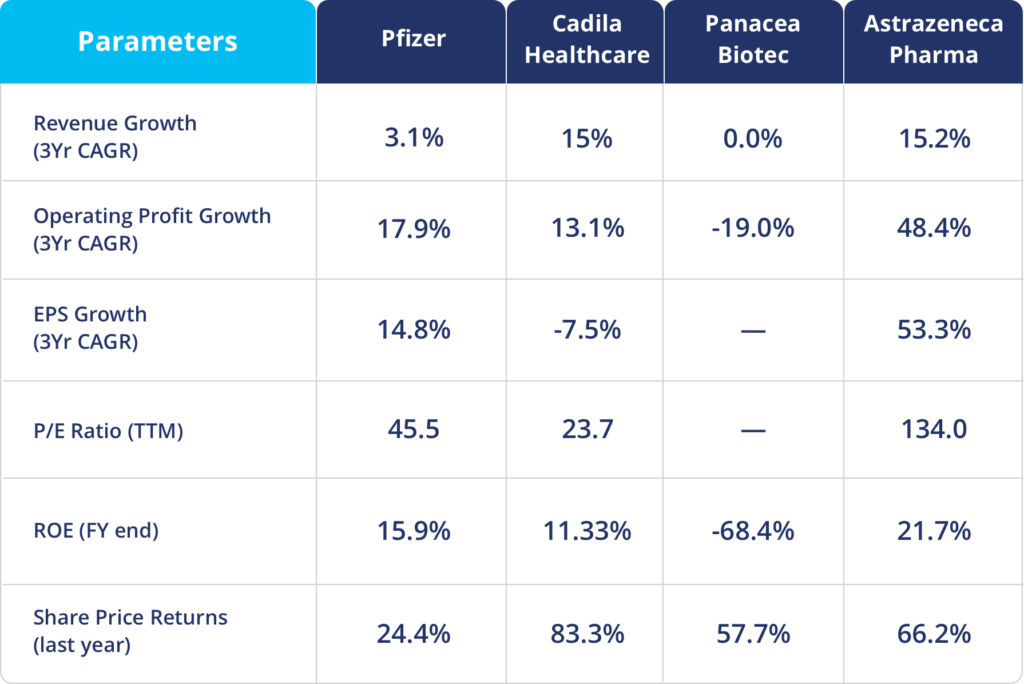
Leading pharma company Serum Institute of India (SII) reported that “it has already manufactured 40 million doses of the vaccine, under the at-risk manufacturing and stockpiling license from DCGI. Also, the company has completed the enrolment of phase-3 clinical trials for the novel coronavirus vaccine-Covishield-in India.” The firm aims to produce the vaccine for India and other middle & low-income countries.
Adar Poonawalla, CEO – SII, said “Based on the current situation and most recent updates on the clinical trials, we are hoping that the AstraZeneca Oxford vaccine will be available towards the end of this year. Apart from AstraZeneca Oxford vaccine and Codagenix, we have associated with multiple institutions worldwide as manufacturing partners for vaccine candidates that are being developed. These include Austria’s Themis along with two others,”.
Another pharma giant Zydus Cadila hopes that clinical trials of ZyCoV-D (its Covid-19 vaccine candidate) would get over in 7 months. The company had begun clinical trials of its Covid-19 vaccine candidate in July 2020.
Pankaj R Patel, Chairman-Zydus Cadila, said “Depending on the study outcomes and if the data is encouraging and the vaccine is found to be effective during the trials, it could take a total of seven months for the trials to be completed and for the vaccine to be launched”.
The Hyderabad-based Bharat Biotech had got approval to conduct Phase 1 & Phase 2 clinical trials of its vaccine named Covaxin at Rohtak’s Post-Graduate Institute of Medical Sciences after its pre-clinical studies exhibited safety and immune response. It has collaborated with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV) to develop the vaccine.
Recently, Dr. Reddy’s, another Hyderabad-based pharma company, has got the regulatory nod to conduct the adaptive phase 2/3 human clinical trial for the Russian Sputnik V vaccine in India. The firm confirmed that the vaccines have reached and clinical trials will begin shortly. Also, Gamaleya National Research Institute of Epidemiology and Microbiology and the Russian Direct Investment Fund have announced that the said vaccine was found to be 92% effective in safeguarding individuals against the coronavirus.
Indian Immunologicals, a subsidiary of the National Dairy Development Board (NDDB), has partnered with Australia’s Griffith University to develop the Covid-19 vaccine. Other pharma companies like Mynvax and Biological E, too, are endeavouring to develop Covid-19 vaccines.
Meanwhile, Pfizer Inc., the US-based pharma giant, reported that its first set of Phase 3 clinical trials of the Covid-19 vaccine provides the initial evidence that its vaccine is 90% effective to prevent Covid-19. Similarly, Moderna, yet another US-based pharma giant, announced that its Phase 3 data of clinical trials state that its Covid-19 vaccine is 94.5% effective in preventing Covid-19.
Are there any constraints?
In India, the manufacturing cost of drugs, including vaccines, is relatively low owing to inexpensive labour and the presence of extensive manufacturing facilities. From a distribution standpoint, it is necessary to offer the vaccine at reasonable prices so that even the people at the grassroots level could get its benefit.
Chandrakant Lahariya, a vaccinologist & public health expert, commented that “A [coronavirus] vaccine developed in India would be far cheaper than a vaccine developed anywhere else. The affordability of a Covid-19 vaccine is paramount; if doses are prohibitively expensive, it could hamper distribution, prolonging the pandemic and costing lives.
SII envisages that it will offer one dose of vaccine at $3 to India and other emerging economies. As against this, Pfizer vaccine, which is getting manufactured in the USA and Germany, would be offered at $19.50 per dose and Moderna would charge in the range of $32-$37 for one dose of vaccine.
Apart from the price considerations, the storage of the Covid vaccine is another impediment in its widespread adoption. The Pfizer vaccine for coronavirus has a complicated super-cold storage requirement i.e. it needs to be stored at -70 degree Celsius. This would become a challenge not only in poor countries or rural missions in India where cold chain facilities are limited but also become a constraint for the most advanced hospitals in the USA. As compared to this, Moderna’s vaccine can be conveniently stored at -20 degrees celsius.
Way forward
As per the US FDA norms, a vaccine needs to be at least 50% effective to meet the approval. Compare that with the 90% effective Covid vaccine, which is very promising and a great achievement in a record-breaking time, that too for the entire humanity. However, any conclusive evidence would be drawn only after the scientific peer-review of the Phase 3 results gets over.
Currently, it’s too early to develop any conclusion. Even as researchers continue to race through clinical trials, they might soon come up with a version that requires less or no refrigeration at all.
Fundamental Analysis